Shelly | September 9, 2022
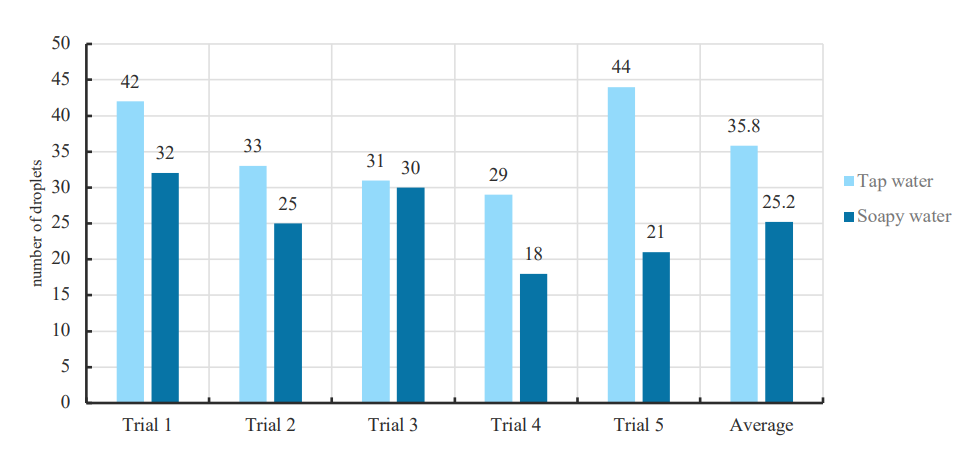
Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area due to the cohesive nature of their molecules. Here comes a question, how does soap change the water’s ability to stay on a penny? When we wash dirty dishes or clothes, for water to flow more easily into the small spaces to clean thoroughly, you need to decrease its surface tension. So, we can develop the hypothesis that adding soap can decrease the surface tension of the water.
